News
Press Releases
Despite External Challenges, WuXi AppTec Maintained Stable Operations in First Half of 2024; Revenue and Profit of the Second Quarter Both Steadily Improved QoQ as Expected, with Revenue Up 16.0% QoQ and Adjusted Non-IFRS Net Profit Up 28.5% QoQ
2024/07/29
- Revenue Reached RMB9,259 Million in the Second Quarter, Excluding COVID-19 Commercial Projects, Up 0.3% Year-over-Year
- Revenue Reached RMB17,241 Million for the First Half, Excluding COVID-19 Commercial Projects, Down 0.7% Year-over-Year
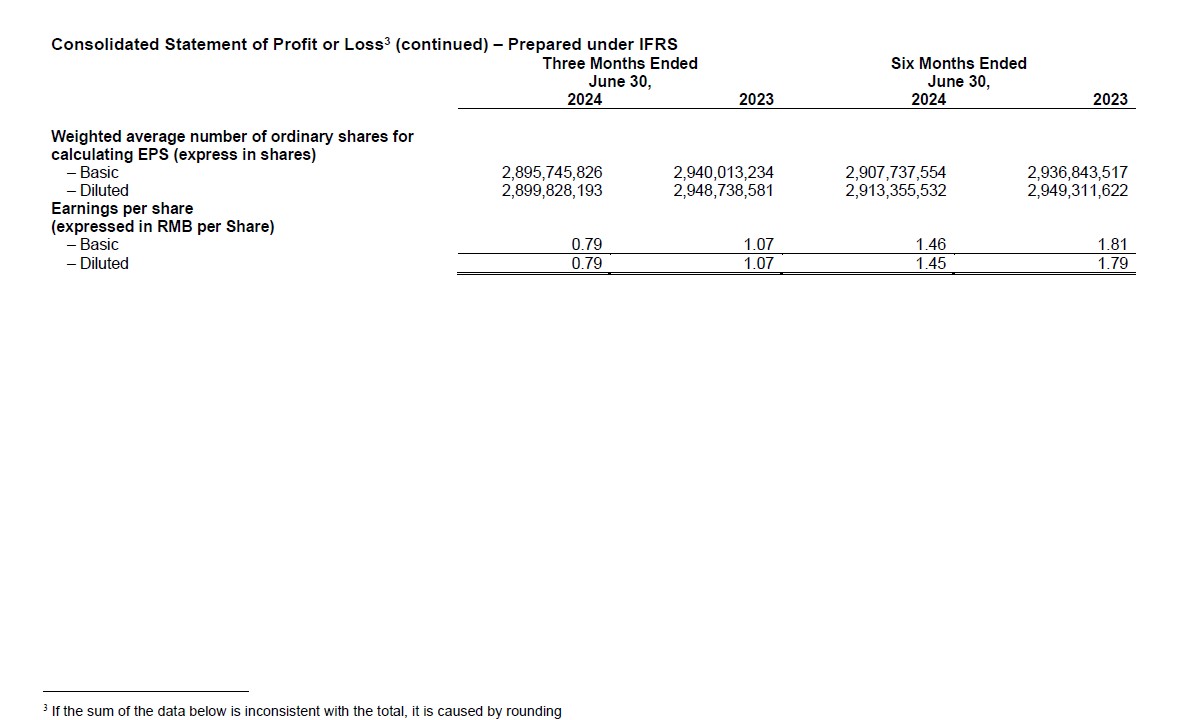
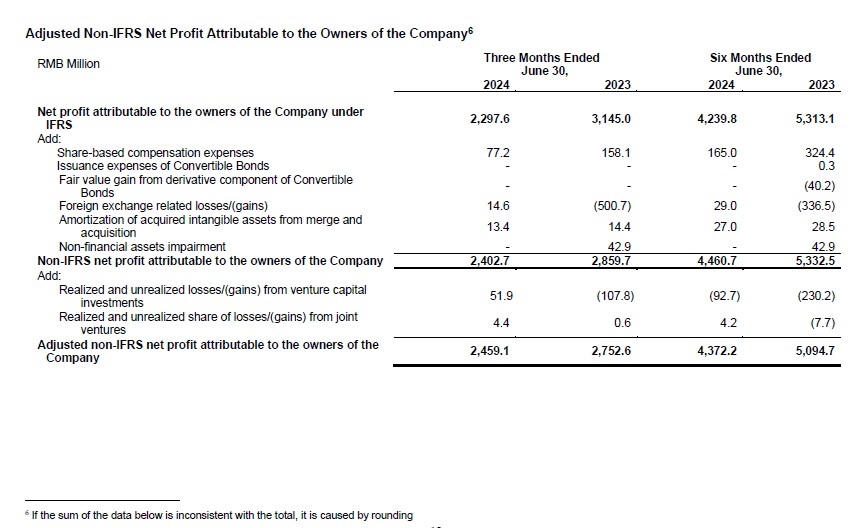
- Net Profit Attributable to the Owners of the Company Reached RMB4,240 Million, and Diluted Earnings per Share (EPS) of RMB1.45 for the First Half
- Adjusted Non-IFRS Net Profit Attributable to the Owners of the Company Reached RMB4,372 Million, and Adjusted Non-IFRS Diluted EPS of RMB1.50 for the First Half
- Excluding COVID-19 commercial projects, Operating Cash Flow Up 48.3%/RMB1.50 billion Year-over-Year for the First Half
(SHANGHAI, July 29, 2024) — WuXi AppTec (stock code: 603259.SH / 2359.HK), a global company that provides a broad portfolio of R&D and manufacturing services that enable companies in the pharmaceutical and life sciences industry, today announced its financial results for the first half ending June 30, 2024 (“Reporting Period”):
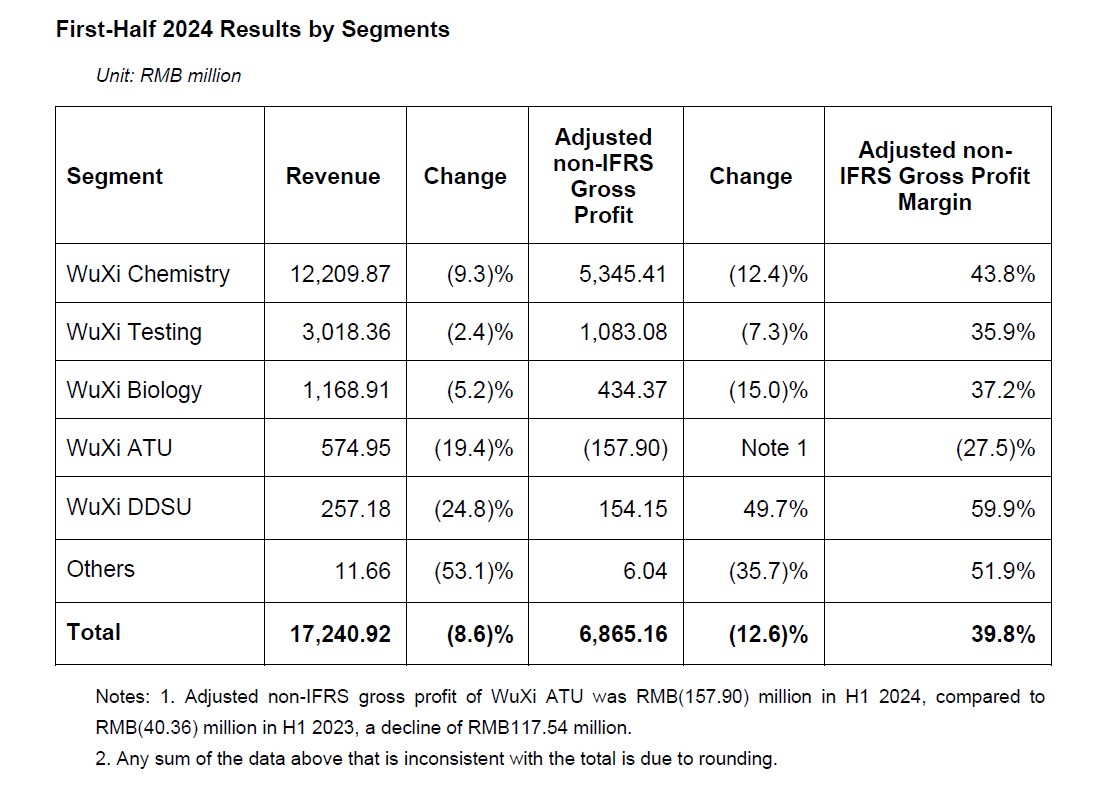
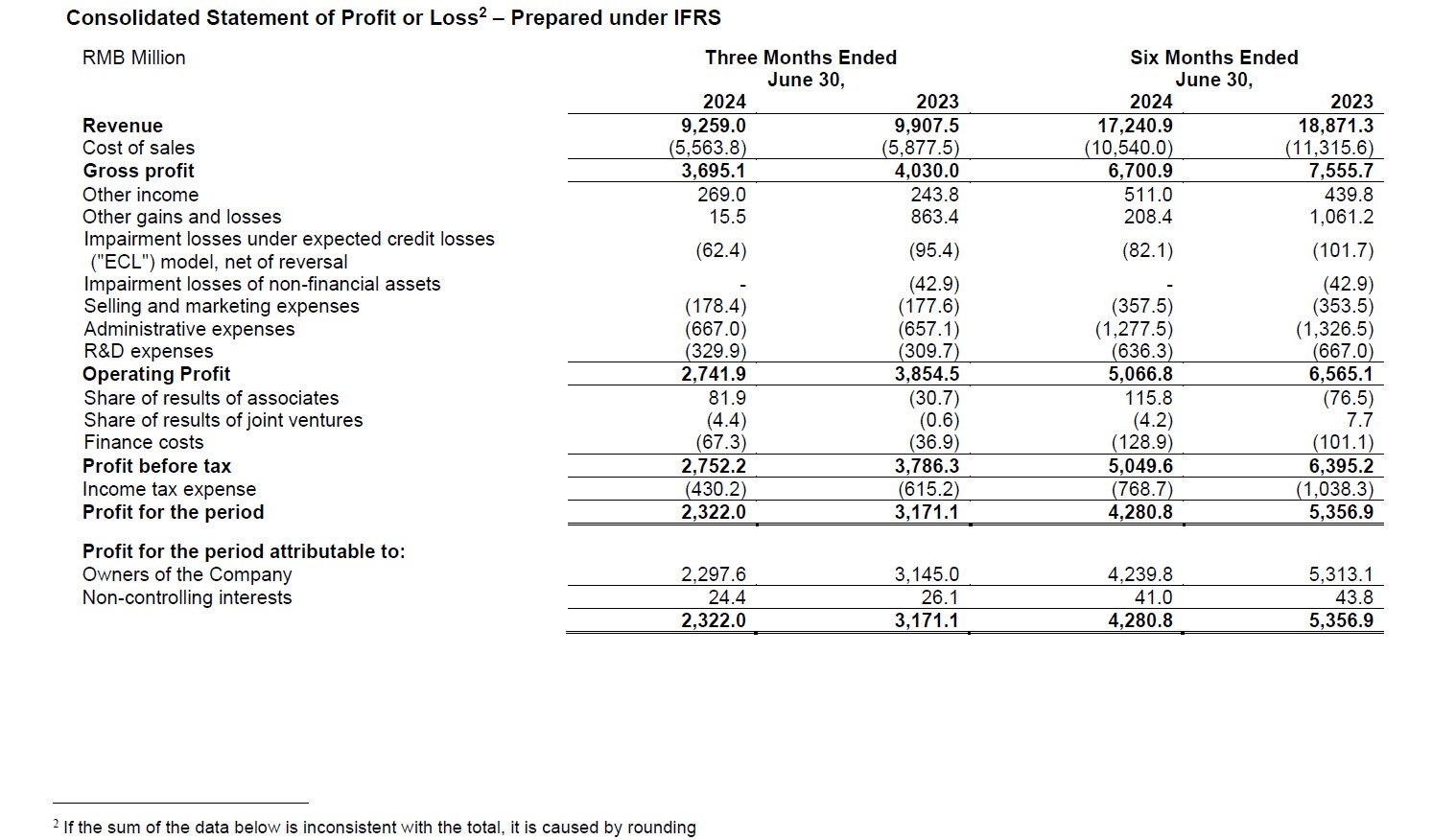
- For the first half of 2024, revenue reached RMB17,241 million, excluding COVID-19 commercial projects, revenue down 0.7% year-over-year.
- Adjusted non-IFRS gross profit reached RMB6,865 million. Adjusted non-IFRS gross profit margin was 39.8%.
- Net profit attributable to the owners of the Company was RMB4,240 million; diluted EPS was RMB1.45. Adjusted non-IFRS net profit attributable to the owners of the Company was RMB4,372 million; adjusted diluted non-IFRS EPS was RMB1.50.
- Excluding COVID-19 commercial projects, operating cash flow grew 48.3%/RMB1.50 billion year-over-year for the first half of 2024.
- In the first half of 2024, we added over 500 new customers in addition to maintaining the existing base of over 6,000 active customers. Demand from customers across regions continued to grow.
- As of June 30, 2024, backlog achieved RMB43.10 billion, growing 33.2% year-over-year excluding COVID-19 commercial projects.
- During the Reporting Period, revenue from the top 20 global pharmaceutical companies was RMB6.59 billion, growing 11.9% year-over-year excluding COVID-19 commercial projects.
- The sustained and steady business growth is attributed to our unique fully integrated Contract Research, Development and Manufacturing Organization (CRDMO) platform. WuXi Chemistry’s small molecule D&M pipeline has maintained rapid growth, with a total of 644 new molecules added in the Reporting Period. As of June 30, 2024, our small molecule D&M pipeline reached 3,319 molecules, among which 14 commercial and phase III projects were added during the Reporting Period.
- In January 2024, total reactor volume of Solid Phase Peptide Synthesizer increased to 32,000L. In May 2024, we announced the groundbreaking of the new R&D and manufacturing site in Singapore.
- Our unique integrated CRDMO business model continues to meet customer demand. The Company keeps investing in D&M capacity, and expects D&M capex to increase more than 50% year-over-year in 2025.
- As an enabler of innovation and a trusted partner and contributor to the global pharmaceutical and life sciences industry, the Company actively promotes sustainability and enhances our global ESG leadership. The Company joined the United Nations Global Compact (UNGC) in 2024, and was named to FTSE4Good Index consecutively in 2023 and 2024. Our outstanding ESG performance has also been widely acknowledged by major global ESG rating agencies, including MSCI, CDP, EcoVadis, S&P and Sustainalytics.
Management Comment
Dr. Ge Li, Chairman and CEO of WuXi AppTec, said, "Despite external challenges, both our revenue and profit in the second quarter of 2024 steadily improved quarter-over-quarter as expected. We achieved backlog of RMB 43.10 billion, growing 33.2% year-over-year excluding COVID-19 commercial projects.”
“The Company's performance in the first half of 2024 again demonstrated that WuXi AppTec's unique integrated CRDMO business model can effectively meet the growing demand of customers worldwide. It enables the Company to closely follow scientific innovations, develop distinct industry insights, instantly seize opportunities in new molecules as they rise, and continue to drive solid business growth. We aim to deliver revenue of RMB 38.3-40.5 billion and free cash flow of RMB 4-5 billion in 2024, while adjusted non-IFRS NPM is expected to remain at a similar level as last year. Although the recently proposed U.S. legislation may create short-term uncertainty for the Company, our customers and the global pharmaceutical and life sciences industry, WuXi AppTec remains steadfast in ‘doing the right thing and doing it right’ and committed to continuously enhancing our capabilities and capacity as we support the industry and our customers to bring groundbreaking therapies to patients around the world.”
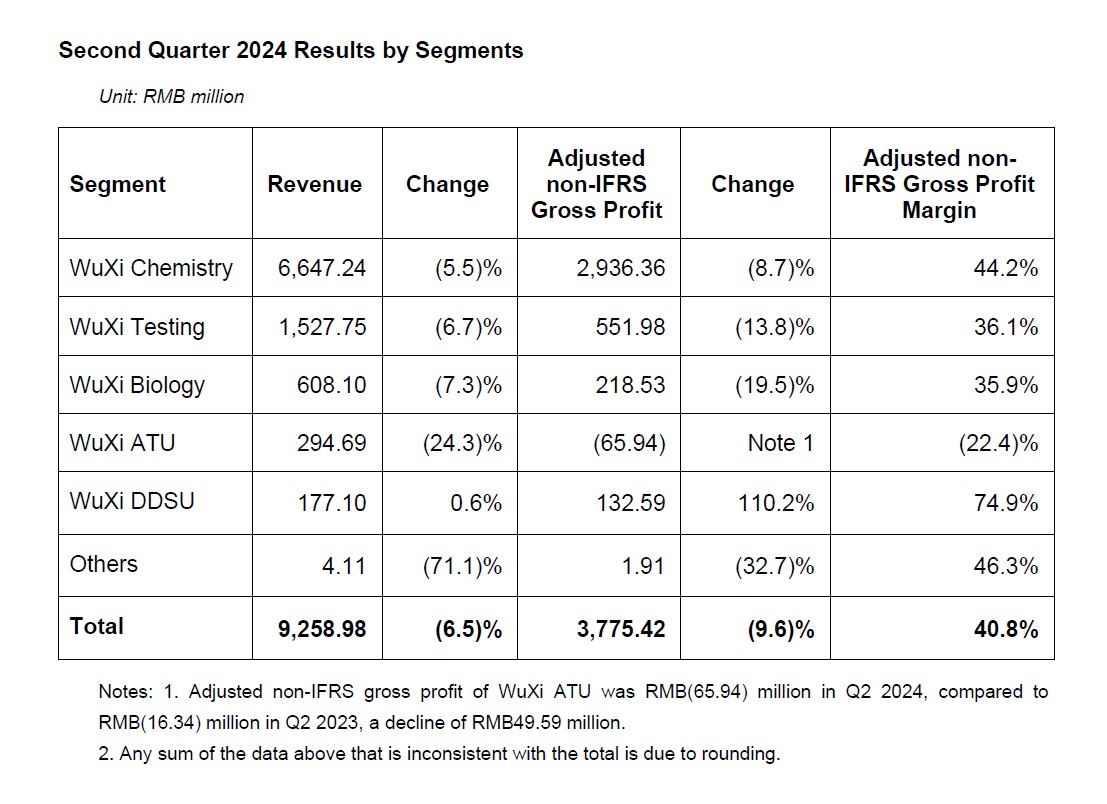
Business Performance by Segments
- WuXi Chemistry: CRDMO Business Model Drives Continuous Growth
- Despite external challenges, H1 revenue of WuXi Chemistry reached RMB12.21 billion, growing 2.1% year-over-year excluding COVID-19 commercial projects. H1 adjusted non-IFRS gross profit margin was 43.8%. With the continued business growth in the second half, full-year gross profit margin is expected to keep flat as last year.
- Small molecule drug discovery services (“R”) continues to generate downstream opportunities. In the past 12 months, we successfully synthesized and delivered more than 450,000 new compounds to customers, and resulted in 7% year-over-year growth. Through our “follow-the-customer” and “follow-the-molecule” strategies, we established trusted partnerships with our customers globally, supporting the sustainable growth of our CRDMO business. The number of molecules converted from R to D&M continued to grow.
- Small molecule development and manufacturing (D&M) services remains strong.
i. H1 revenue of small molecule D&M services reached RMB7.39 billion. Excluding COVID-19 commercial projects, H1 revenue was down 2.7% year-over-year on top of the strong base of over 50% year-over-year growth in the first half of 2023, and full-year revenue is expected to maintain positive growth.
ii. Small molecule CDMO pipeline continued to expand. In the first half, 644 new molecules were added to the small molecule D&M pipeline. As of June 30, 2024, our small molecule D&M pipeline reached 3,319 molecules, including 67 commercial projects, 74 in phase III, 353 in phase II and 2,825 in phase I and pre-clinical stages, among which 14 commercial and phase III projects were added during the Reporting Period.
iii. In May 2024, we announced the groundbreaking of the new R&D and manufacturing site in Singapore.
- Specifically, TIDES business (mainly oligo and peptides) sustains rapid growth.
i. H1 revenue of TIDES grew strongly by 57.2% year-over-year to RMB2.08 billion. As of June, 30, 2024, TIDES backlog grew 147% year-over-year.
ii. The number of TIDES D&M customers increased 25% year-over-year to 151, and the number of TIDES molecules increased 39% year-over-year to 288.
iii. In January 2024, the total reactor volume of solid phase peptide synthesizers increased to 32,000L.
- WuXi Testing: Drug Safety Evaluation Service & Site Management Organization (SMO) Maintain Leading Positions
- H1 revenue of WuXi Testing reached RMB3.02 billion. Adjusted non-IFRS gross profit margin was 35.9%.
- H1 revenue of lab testing services was down 5.4% year-over-year to RMB2.12 billion. Among which, revenue from drug safety evaluation services was down 6.3% year-over-year due to market impact, while we maintained an industry leading position in the Asia-Pacific region.
- In the first half, the Qidong and Chengdu facilities both received the National Medical Products Administration (NMPA) and Organization for Economic Co-operation and Development (OECD) GLP qualifications. The Suzhou facility was reviewed for the first time by the Japan Pharmaceuticals and Medical Devices Agency (PMDA) for on-site audit and successfully passed.
- New modality business continued to develop, while new vaccine capability continued to improve, and market share of nucleic acids, conjugates, and mRNA further expanded.
- H1 revenue of clinical CRO & SMO grew 5.8% year-over-year to RMB0.89 billion. Among which, SMO revenue grew 20.4% year-over-year, maintaining industry leading position in China. In the Reporting Period, SMO supported 31 new drug approvals for customers, and Clinical CRO enabled our customers to obtain 14 IND approvals.
- SMO business continued steady growth, maintaining significant advantages in multiple therapeutic areas (cardiovascular disease, ophthalmology, rheumatology, central nervous system, endocrinology, medical aesthetics and rare tumors, etc.).
- WuXi Biology: New Modality Business Drives Growth; WuXi Biology Platform Continues to Generate Downstream Opportunities
- H1 revenue of WuXi Biology reached RMB1.17 billion. Adjusted non-IFRS gross profit margin was 37.2%.
- The Company focused on improving capabilities related to new modalities. In the Reporting Period, revenue from new modalities grew 8.1% year-over-year, contributing 29.0% of WuXi Biology revenue.
- Number of customers and projects served by the nucleic acid platform continued to increase. Cumulatively, the Company provided services to 260+ customers, and successfully delivered 1,200+ projects since 2021.
- The Company proactively built capabilities to collaboratively develop membrane proteins and peptides, leading to remarkable increase in business volume of related protein production, screening and subsequent validation services.
- The Company further integrated resources of the in vivo pharmacology platform, and continued to improve platform capabilities and efficiency. The Company also fully leveraged the advantage of the one-stop service platform with in vitro & in vivo synergy to further gain market share in metabolic, cardiovascular and neurological areas, and the number of customers served grew 30%+ year-over-year.
- WuXi Biology continued to generate downstream opportunities and contributed over 20% of the Company’s new customers.
- WuXi ATU: Commercial and Existing Clinical Stage Projects Continue to Advance; Revenue and Profit below Expectation due to Proposed U.S. Legislation
- H1 revenue of WuXi ATU reached RMB0.57 billion. Adjusted non-IFRS gross profit margin was (27.5)%. Primarily due to: 1) the completion of high-margin projects in 2023; commercial projects are still in early stage of ramping up; 2) certain projects were delayed, or cancelled due to customers’ pipeline prioritization or funding issue; as well as insufficient new business wins due to the proposed U.S. legislation.
- The Company continues to improve our CTDMO integrated enabling platform. As of June 30, 2024, we provided development, testing and manufacturing services for 64 projects, including 2 commercial projects, 5 Phase III projects (2 projects in BLA preparation stage), 8 Phase II projects and 49 pre-clinical and Phase I projects, among which, the world’s first innovative TIL-based therapy was approved by the U.S. Food and Drug Administration (FDA) in February 2024.
- We are preparing for BLA filing to manufacture the lentiviral vector (LVV) used in a commercial CAR-T product. We completed process performance qualification (PPQ), started post-PPQ manufacturing, and expect to file pre-approval submission (PAS) to FDA in the second half of 2024. Moreover, we expect to complete PPQ in the second half of 2024 and file PAS to FDA in the first half of 2025 for a blockbuster commercial CAR-T product.
This release provides a summary of the results and is not intended to be a comprehensive report. For additional information, please refer to the WuXi AppTec 2024 Interim Results Presentation and 2024 Interim Report disclosed on the Company’s official website, as well as the 2024 Interim Report and other relevant announcements published on the websites of the Shanghai Stock Exchange (www.sse.com.cn) and the Stock Exchange of Hong Kong (www.hkexnews.hk), and the designated media for dissemination of the relevant information. Investors are advised to exercise caution and be aware of the investment risks in trading Company shares.
All financial information disclosed in this press release is prepared based on International Financial Reporting Standards (IFRS), in currency of RMB.
The 2024 Interim Report of the Company has not been audited.
About WuXi AppTec
As a global company with operations across Asia, Europe, and North America, WuXi AppTec provides a broad portfolio of R&D and manufacturing services that enable the global pharmaceutical and life sciences industry to advance discoveries and deliver groundbreaking treatments to patients. Through its unique business models, WuXi AppTec’s integrated, end-to-end services include chemistry drug CRDMO (Contract Research, Development and Manufacturing Organization), biology discovery, preclinical testing and clinical research services, advanced therapies CTDMO (Contract Testing, Development and Manufacturing Organization), helping customers improve the productivity of advancing healthcare products through cost-effective and efficient solutions. WuXi AppTec received an AA ESG rating from MSCI for the third consecutive year in 2023 and its open-access platform is enabling more than 6,000 customers from over 30 countries to improve the health of those in need – and to realize the vision that "every drug can be made and every disease can be treated." Please visit: http://www.wuxiapptec.com
Forward-Looking Statements
This press release may contain certain “forward-looking statements” which are not historical facts, but instead are predictions about future events based on our beliefs as well as assumptions made by and information currently available to our management. Although we believe that our predictions are reasonable, future events are inherently uncertain and our forward-looking statements may turn out to be incorrect. Our forward-looking statements are subject to risks relating to, among other things, the ability of our service offerings to compete effectively, our ability to meet timelines for the expansion of our service offerings, our ability to protect our customers’ intellectual property, unforeseeable international tension, competition, the impact of emergencies and other force majeure. Our forward-looking statements in this press release speak only as of the date on which they are made, and we assume no obligation to update any forward-looking statements except as required by applicable law or listing rules. Accordingly, you are strongly cautioned that reliance on any forward-looking statements involves known and unknown risks and uncertainties. All forward-looking statements contained herein are qualified by reference to the cautionary statements set forth in this section. All information provided in this press release is as of the date of this press release and are based on assumptions that we believe to be reasonable as of this date, and we do not undertake any obligation to update any forward-looking statement, except as required under applicable law.
Use of Non-IFRS and Adjusted Non-IFRS Financial Measures
We provide non-IFRS gross profit and non-IFRS net profit attributable to the owners of the Company, which exclude share-based compensation expenses, issuance expenses of convertible bonds, fair value gain or loss from derivative component of convertible bonds, foreign exchange-related gains or losses, amortization of acquired intangible assets from merge and acquisition, non-financial assets impairment, talent incentive and retention expenses funded by cash donation from shareholders, etc. We also provide adjusted non-IFRS net profit attributable to the owners of the Company and earnings per share, which further exclude realized and unrealized gains or losses from our venture capital investments and joint ventures. Neither is required by, or presented in accordance with IFRS.
We believe that the adjusted financial measures used in this press release are useful for understanding and assessing our core business performance and operating trends, and we believe that management and investors may benefit from referring to these adjusted financial measures in assessing our financial performance by eliminating the impact of certain unusual, non-recurring, non-cash and non-operating items that we do not consider indicative of the performance of our core business. Such adjusted non-IFRS net profit attributable to the owners of the Company, the management of the Company believes, is widely accepted and adopted in the industry the Company is operating in. However, the presentation of these adjusted non-IFRS financial measures is not intended to be considered in isolation or as a substitute for the financial information prepared and presented in accordance with IFRS. You should not view adjusted results on a stand-alone basis or as a substitute for results under IFRS, or as being comparable to results reported or forecasted by other companies.

